By Kian Talaei, MEng ’20 (IEOR)
Have you ever wondered how Theranos and its “revolutionary” technology slid past FDA regulations? New technology in general poses difficulties for regulators. With advances in artificial intelligence (AI) and machine learning (ML) in the medical technology industry (medtech), what will happen to FDA regulations in the near future? Medtech is in a period of groundbreaking change, with data at the epicenter of that change. This change, however, brings a level of uncertainty for the FDA as they approach regulations. If the FDA is already encountering difficulties regulating “locked” AI/ML-based software as a medical device (SaMD), how will they handle adaptive SaMDs, whose algorithms “adapt” or change with new data introduced in the system? In the following, I introduce a regulatory solution to solve the huge challenge around regulating AI/ML-based innovations. Theranos’s blood diagnosis machine was the first and biggest scandal in the dawn of advances in the medtech industry. How can a company raise so much money and survive so long without proper FDA backing? According to Elizabeth Holmes, FDA approval was only voluntary and her decision to receive FDA approval for the Herpes test was based on her decision that “FDA approval is an important step towards validating the company’s technology” (Friedman 2015). The FDA’s approach towards the Edison machine was highly unethical and nearly put the lives of millions in danger. The regulatory procedures towards the company in addition to the company’s misreporting of device capabilities should be a wake-up call for regulatory agencies to understand the necessary guidelines they need to uphold at all times. Artificial intelligence, “the science of making intelligent machines,” and machine learning are revolutionizing many industries and are at the forefront of transformation in the medtech field (McCarthy 2017). Afshin Bazargan, a research and development director of Medtronic, stated how “artificial intelligence and machine learning and the data behind them are becoming extremely important in the medical device industry, especially in our approaches to concept generation. There is a new era of technology.” Within this new era of growth there are two underlying regulatory distinctions to discuss. There are two types of AI/ML SaMDs: “locked” and “adaptive” (Babic et al 2019). Locked AI/ML-based SaMDs are those in which the algorithm for the device is invariable when sent for FDA approval; by contrast, adaptive refers to devices in which the algorithm “learns” based on new data. AI/ML-based SaMD are already assisting in early cancer detection using radiology images, early heart disease diagnosis using EKG data, and medication dosage selection using diagnostics and gene data (“Software as a Medical Device”). Based on the efficiency and impact of AI technology, our approach to healthcare will move towards a future of adaptive and data driven medical devices (Figure 1).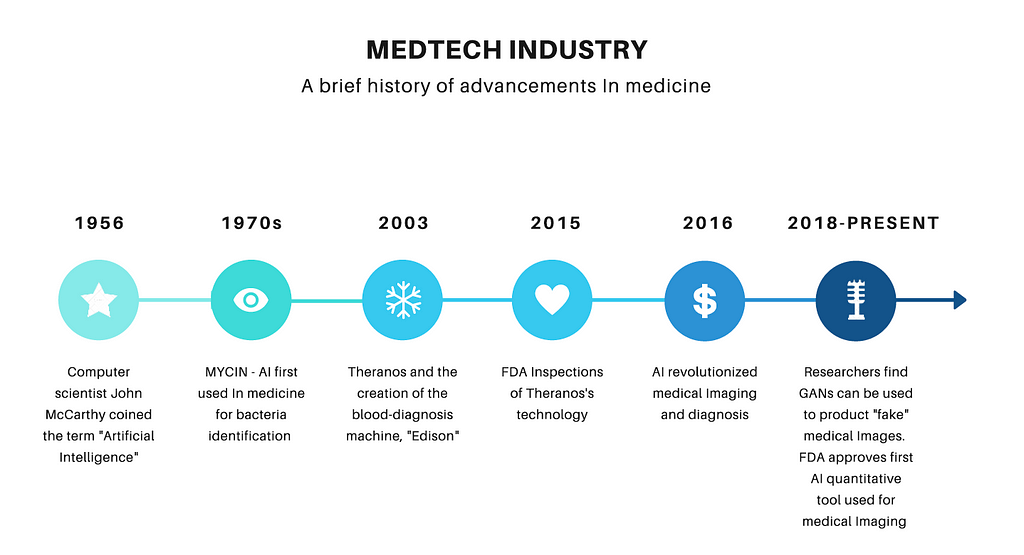
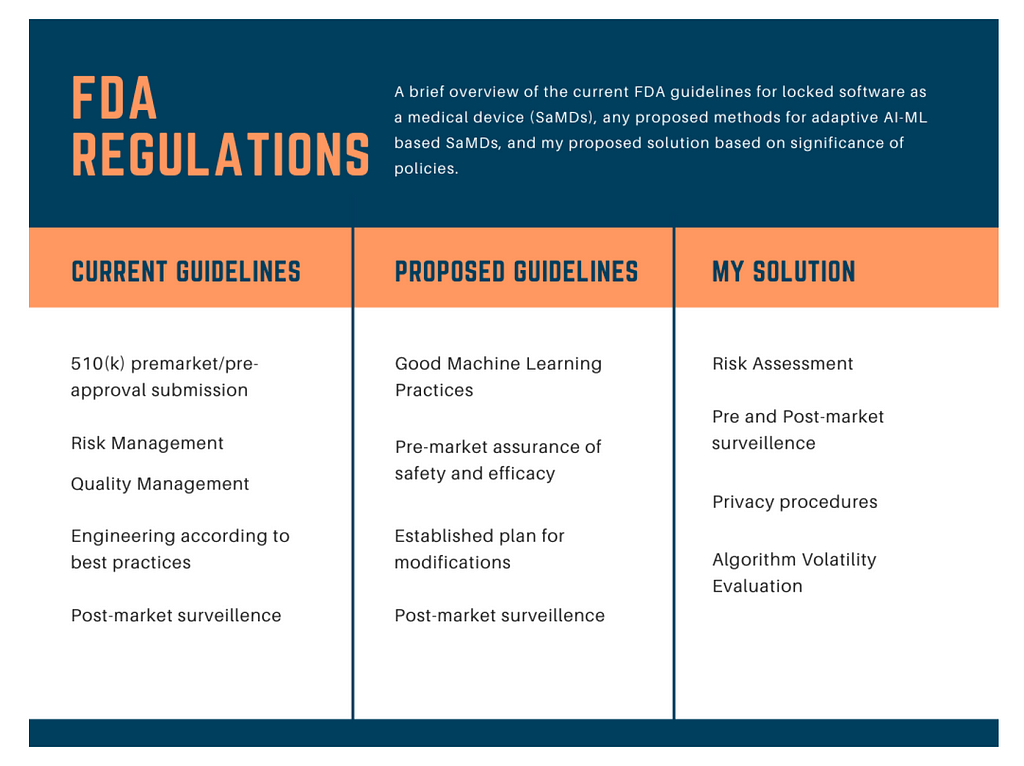
Advances in technology must always prioritize public safety and privacy.As depicted in Figure 2, current FDA regulations address locked SaMDs through regular pre-market approval, but adaptive devices, which frequently re-optimize to improve health, are not within the scope of current regulations (AI and ML in software 2019).
About the author:
Kian Talaei is a current Master of Engineering student at UC Berkeley, studying Industrial Engineering Operations Research (IEOR) with a focus on Data Analytics. Kian is an all-encompassing student interested in economics, medicine, and tech. Connect with Kian.References:
- “Artificial Intelligence and Machine Learning in Software.” U.S. Food and Drug Administration, FDA, 2019.
- Chuquicusma, et al. “How to Fool Radiologists with Generative Adversarial Networks? A Visual Turing Test for Lung Cancer Diagnosis.” ArXiv.org, 9 Jan. 2018, arxiv.org/abs/1710.09762.
- “FDA Selects Participants for New Digital Health Software Precertification Pilot Program.” U.S. Food and Drug Administration, FDA, 2017.
- Friedman, Lauren F. “Controversial Multibillion-Dollar Health Startup Theranos Just Got a Huge Seal of Approval from the US Government.” Business Insider, Business Insider, 2 July 2015.
- McCarthy, John. “WHAT IS ARTIFICIAL INTELLIGENCE.” Stanford Computer Science , 12 Nov. 2017.
- “Proposed Regulatory Framework for Modifications to Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device.” U.S. Food and Drug Administration, 2019.
- “‘Software as a Medical Device’: Possible Framework for Risk Categorization and Corresponding Considerations.” IMDRF Software as a Medical Device (SaMD) Working Group, 18 Sept. 2014.
Op-ed: The role of the FDA in the future of medtech innovation was originally published in Berkeley Master of Engineering on Medium, where people are continuing the conversation by highlighting and responding to this story.